Describe the Role of Energy in Chemical Reactions
Energy is absorbed in a chemical reaction that results in the formation of a new substance. Even reactions that release energy need a boost of energy in order to begin.
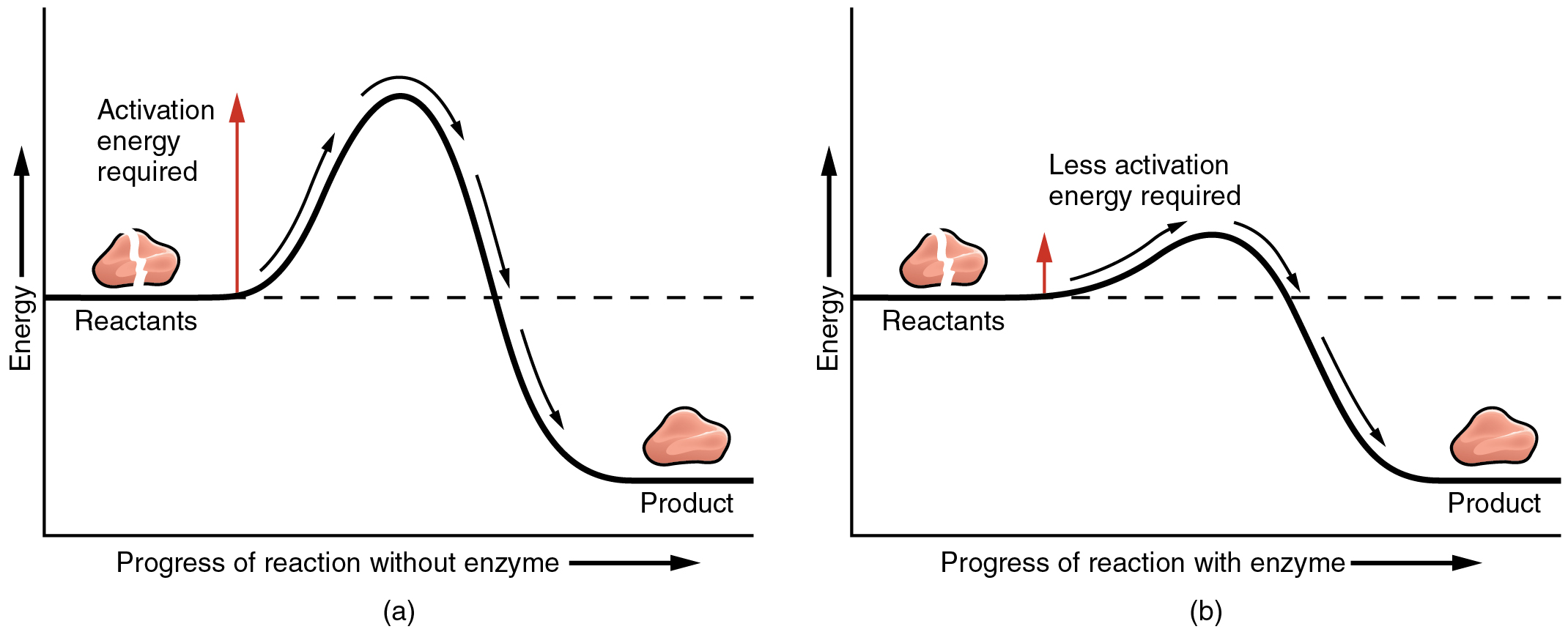
Chemical Reactions Anatomy And Physiology
Both types of reactions involve exchanges not only of matter but of energy.

. Energy is used to break bonds in reactants and energy is released when new bonds form in products. In chem reactions atoms of the reactants recombine to form new substances in the products. The energy needed to start a chemical reaction is called activation energy.
So there are two ways to produce the energy in a reaction and they are either exothermic reaction producing energy or endothermic reaction consuming energy from the surroundings. Discuss how chemical reactions play a role in energy transfer Scientists use the term bioenergetics to discuss the concept of energy flow link through living systems such as cells. This means that it is similar to molecules.
Chemical changes are not easily reversed. A substances particles are changed during a chemical reaction. Energy plays a key role in chemical processes.
When chemical bonds break energy is absorbed it takes energy to break a chemical bond. Living organisms must take in energy via food nutrients or sunlight in order to carry out cellular processes. Energy is absorbed to break bonds and energy is evolved as bonds are made.
Mass is conserved in a chemical reaction though the molecule count may. Energy also has a role to play in the entropy or randomness of a chemical system by which we mean a quantity of substance or substances such as a reaction mixture. Ssignment 6 - Chapter 3 Saved Help Save Exit Complete the following paragraph to describe the role of ATP.
The balanced chemical equation represents. Please update your bookmarks accordingly. In chemical reactions atoms of the reactants recombine to form new substances in the products.
Living organisms must take in energy via food nutrients or sunlight in. Imagine you are building a brick wall. Covers the role of energy in chemical reactions.
Cellular processes such as the building and breaking down of complex molecules occur through stepwise chemical reactions. Metabolism is a combination of chemical reactions that are spontaneous and release energy and chemical reactions that are non-spontaneous and require energy in order to proceed. All chemical reactions need energy to get started.
The Role of Energy in Chemical Reactions. Energy exists in packets called quanta. 9 What is the role of a catalyst in a chemical reaction briefly describe how it accomplishes its role.
Chemical reactions require a sufficient amount of energy to cause the matter to collide with enough precision and force that old. There is almost always some transfer between the thermal and chemical energy accounts as well as an exchange of thermal energy via heating Q between the system and surroundings. The reaction is enabled when there is an external path for electric current and ceases when that path is broken.
There is almost always some transfer between thermal and chem energy accounts as well as an exchange of thermal energy via heating Q between the system and surroundings. Chemical reactions provide a way to get at primary energy in fuels both primary fuels like coal oil and natural gas and also secondary fuels like molecular hydrogen and gasoline. In a chemical reaction that causes the decomposition of a substance energy is released.
A how enzymes affect reaction rates B how enzymes affect activation energy C how enzymes interact with reactants and how D activators and competitive and non-competitive inhibitors interact with enzymes. According to the modern view of chemical reactions bonds between atoms in the reactants must be broken and the atoms or pieces of molecules are reassembled into products by forming new bonds. 10 What changes when a catalyst on a chemical reaction is to provide a new reaction pathway that results in a different.
Being that the motivation for electrons to move through a cell is chemical in nature the amount of voltage electromotive force generated by any cell will be specific to the particular chemical reaction for that cell type. The Role of Energy in Chemical Reactions. The role of energy.
12 What are two ways a catalyst affect a chemical reaction. Describe the role of enzymes in chemical reactions in detail. In a chemical reaction energy is used for breaking the bonds of the reactants also energy is used in formation of new bonds.
Like the combustion reaction in a furnace some chemical reactions require less energy to break bonds in reactants than is released when bonds form in products. You cannot have half a molecule and similarly you can have any whole number of quanta of energy but. Chemical reactions require a sufficient amount of energy to cause the matter to collide with enough precision and force that old chemical bonds can be broken and new ones formed.
Exothermic reactions release heat and light into their surroundings. Adenosine diphosphase ATP or provides energy for nerve impulse conduction muscle contraction and many other chemical reactions necessary for life. The energy change in a chemical reaction is due to the difference in the amounts of stored chemical energy between the products and the reactants.
When chemicals react chemical bonds are broken and then restructured. Some chemical reactions release energy and other chemical reactions absorb energy. Covalent nts unstable eBook eferences It is a stable that is comprised of adenine ribose.
In general kinetic energy is the form of energy powering any type of matter in motion. Use relevant course terminology and explain. Activation energy is like the push a child needs to start going down a playground slide.
All chemical reactions involve energy. 11 Why do catalysts increase the rate of chemical reactions. Describe the role of energy in chemical reactions.
Metabolism is a combination of chemical reactions that are spontaneous and release energy and chemical reactions that are non-spontaneous and require energy in order to proceed. We have moved all content for this concept to for better organization. This stored chemical energy or heat content of the system is known as its enthalpy.
Energy changes determine how easily a chemical reaction will occur. The balanced chemical equation represents this process symbolically showing that the atoms in the.
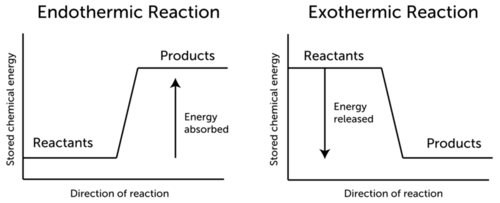
Conservation Of Energy In Chemical Reactions Ck 12 Foundation

Understanding Endothermic And Exothermic Reactions Chemistry Experiments Exothermic Reaction Chemical Reactions
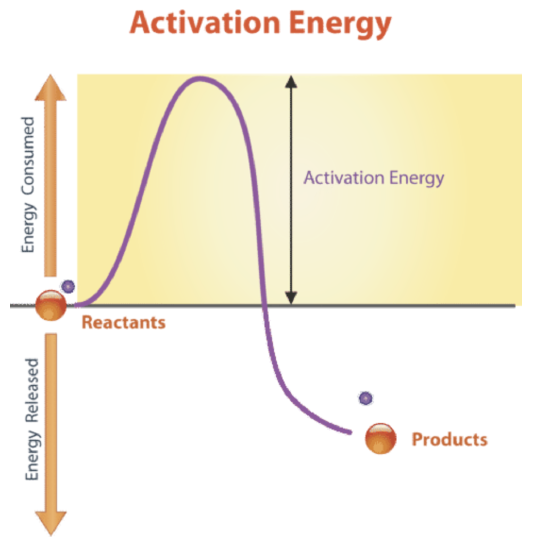
Comments
Post a Comment